Bevacizumab treatment beneficial for patients with severe COVID-19
Published: 2021-02-18
The COVID-19 pandemic has caused a serious health emergency and a global challenge for demand of novel drug development. Drug repurposing has emerged as one way to meet the demand for new COVID-19 treatments.
Severe COVID-19 has several clinical symptoms, for example, acute respiratory distress syndrome (ARDS) and tissue hypoxia in lungs and other organs. Previous research has shown that hypoxia induces vascular endothelial growth factor (VEGF) expression. In addition, VEGF is a potent vascular permeability factor that induces leakiness in COVID-19-infected lung tissues which can further contribute the pulmonary edema, and tissue hypoxia. Drugs that block VEGF could therefore prove beneficial to alleviate severe COVID-19 symptoms.
A recently published clinical study led by Yihai Cao, professor of Vascular Biology at the Department of Microbiology, Tumor and Cell Biology at Karolinska Institute, Stockholm (first authors: Jiaojiao Pang, Feng Xu, Gianmarco Aondio) investigating bevacizumab, a humanized anti-VEGF monoclonal antibody, for treating severe COVID-19. Bevacizumab is a medication used for cancer treatment since the early 2000s. Bevacizumab slows the formation of new blood vessels by inhibiting VEGF. In Sweden, it is registered under the brand names Avastin, Aibintio, MVASI, and Zirabev.
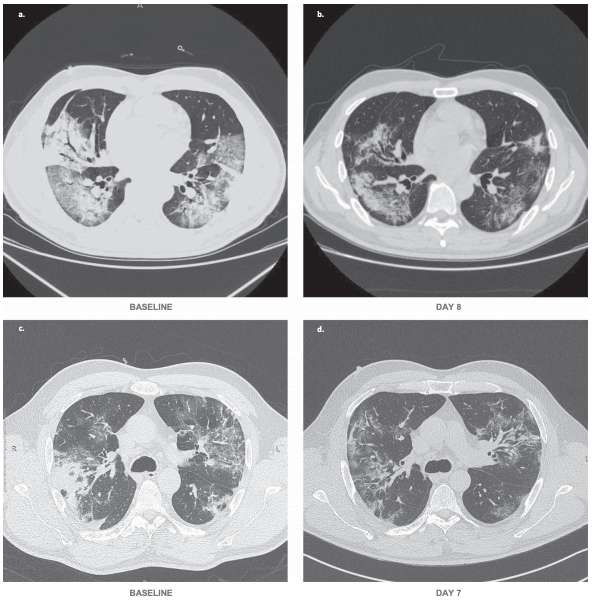
The clinical trial, conducted between February and April 2020 in China and Italy, followed 26 patients over 28 days (inclusion criteria respiratory rate ≥30 times/min, oxygen saturation ≤93% with ambient air, or partial arterial oxygen pressure to fraction of inspiration O 2 ratio (PaO2/FiO2) >100 mmHg and ≤300 mmHg, and diffuse confirmed pneumonia). The results showed that patients who received a combination of bevacizumab and standard care showed markedly improved PaO2/FiO2 ratios at days 1 and 7. Subgroup (Chinese=12 and Italian=14) analysis showed that only the Italian population displayed increases of PaO2/FiO2 ratios at day 7 with statistical significance.
By day 28 of bevacizumab, 92% of patients showed improvement in oxygen-support status, and 65% were discharged from hospital. No patient who received the combination of bevacizumab and standard care showed worsen oxygen-support status or had died after 28 days. In the control group 46% were discharged from hospital by day 28 and three patients had died. Bevacizumab also shortened the duration of oxygen-support to a median of nine days compared with 20 for the standard care group. The researchers describe that patients exhibited reduced lesions shown in CT or X-ray and a normalization of body temperatures. Decreased oxygen supplementation can both improve the health status for individual patients and decrease the healthcare burden.
“Repurposing the existing drugs such as bevacizumab for treating other diseases provides an important avenue for treating patients with COVID-19. Importantly, the risk of these already FDA-approved drugs is minimal because their toxicity profiles are very familiar to clinicians. While developing completely new drugs take years of time for clinical approval, repurposing existing drugs provide a fast, safe, and effective approach for treating Covid patients. In this case, bevacizumab provides an outstanding example of an anticancer drug can reduce mortality of Covid patients. It can convert severe Covid pneumonia into non-life threatening mild disease.“ said prof. Yihai Cao.
In summary, the researchers suggest bevacizumab may be beneficial for patients with severe COVID-19 and randomized control trials are warranted. These results also highlight drug repurposing of VEGF inhibitors as an important future treatment option for severe COVID-19 patients.
Besides the study results, the researchers also shared data and analysis code publicly, enabling their re-use in the future. They published the full SAS analysis code which can be used for analyses of clinical trials of other drugs. Raw data file containing demographic and clinical characteristics, progress of treatment, and laboratory test results for each patient is also available. These data not only allow for re-analyses, but can also be used for comparisons with the outcome of other clinical trials of bevacizumab.
The study was supported by research grants from the European Research Council (ERC) advanced grant ANGIOFAT (project no 250021), the Swedish Research Council, the Swedish Cancer Foundation, the Strategic Research Areas (SFO)–Stem Cell and Regeneration Medicine Foundation, the Karolinska Institutet, the Swedish Children’s Cancer Foundation, the Karolinska Institutet Foundation, the Karolinska Institutet Distinguished Professor Award, the Torsten Soderbergs Foundation, the Maud and Birger Gustavsson Foundation, the Novo Nordisk Foundation–Advance grant, and the Knut and Alice Wallenberg’s Foundation and National Key R& D Program of China (2020YFC0846600) and the Shandong Provincial Key R& D Program (2020SFXGFY03).
Article
DOI: 10.1038/s41467-021-21085-8
Pang, J., Xu, F., Aondio, G., Li, Y., Fumagalli, A., Lu, M., Valmadre, G., Wei, J., Bian, Y., Canesi, M., Damiani, G., Zhang, Y., Yu, D., Chen, J., Ji, X., Sui, W., Wang, B., Wu, S., Kovacs, A., Revera, M., Wang, H., Jing, X., Zhang, Y., Chen, Y. & Cao Y. Efficacy and tolerability of bevacizumab in patients with severe COVID-19. Nature Communications, 12 (814) (2021).
Data
- Data file with demographic and clinical characteristics of the patients as well as data on dynamic changes of PaO2/FiO2 between bevacizumab (treatment) and control groups. DOI: 10.6084/m9.figshare.13482810.v1
- Analysis code in SAS. DOI: 10.6084/m9.figshare.13482834.v1